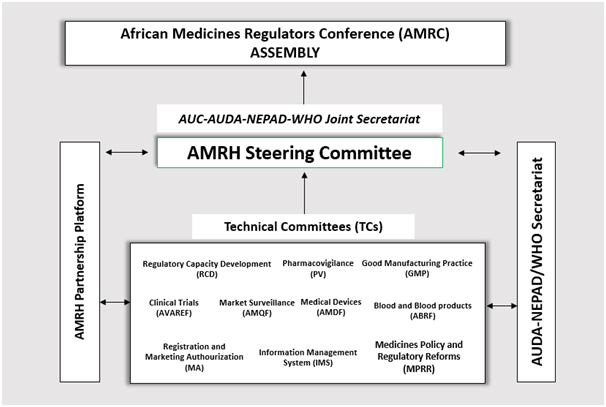
African Medicines Regulatory Harmonization (AMRH) programme started in 2009 as a response to addressing challenges faced by National Medicine Regulatory Authorities (NMRAs) in Africa. These challenges include; weak or non-coherent legislative frameworks, sluggish medicine registration processes and subsequent delayed approval decision, inefficiency and limited technical capacity, among others. This situation translates in to poor access to priority essential medicines by patients and is a contributing factor to over-priced medicines.
The aim of the programme is to facilitate and coordinate the harmonization of medicines regulation and improve access to quality, safe, efficacious and affordable medicines in Africa as part of the broader African Union Framework on Pharmaceutical Manufacturing Plan for Africa (PMPA). To achieve this aim, AMRH works towards improving medicine registration processes and operational inefficiencies, thereby reducing registration times whilst enhancing the quality of the registration decision. The programme is implemented under the Program Delivery and Coordination Directorate.